First Law of Thermodynamics and Mechanical Equivalent of Heat : Joule’s Experiment
First Law of Thermodynamics
The first law thermodynamics is basically the law of conservation of energy, and it used in the analysis of system involving heat and work transfer. In general terms,
the first law of thermodynamics states that the heat and work are mutually convertible
The first law interrelate between heat and work, and cannot be proved mathematically. It is based on experimental observations, and so far no theory violates these observations.
In otherwords
It is observed that when a system is made to undergo a complete cycle then net work is done on or by the system. Consider a cycle in which net work is done by the system. Since energy cannot be created, this mechanical energy must have been supplied from some source of energy. Now the system has been returned to its initial state : Therefore its internal energy is unchanged and hence the mechanical energy has not been provided by the system itself. The only other energy involved in the cycle is the heat which was supplied and rejected in various processes. Hence, by the law of conservation of energy, the net work done by the system is equal to the net heat supplied to the system. The first law of thermodynamics can, therefore, be stated as follows :
When a system undergoes a thermodynamic cycle then the net heat supplied to the system from the surroundings is equal to net work done by the system on its surroundings.
∮ dQ = ∮ dW
where ∮ represents the sum for a complete cycle
The first law of thermodynamics cannot be proved analytically but experimental evidence has repeatedly confirmed its validity, and since no phenomenon has been shown in contradict it, the first law is accepted as a law of nature.
It may be remarked that no restriction was imposed which limited the application of first law to reversible energy transformation. Hence the first law applies to reversible as well as Irreversible transformations : For non-cyclic process, a more general formulation of first law of thermodynamics is required. A new concept which involves a term called internal energy fulfills this need.
First Law of a Closed System Undergoing a Cycle :
The first law of thermodynamics states that during any cycle a system undergoes the cyclic integral of heat is equal to the cyclic integral of the work.
 |
| Fig.1 First Law of Thermodynamics |
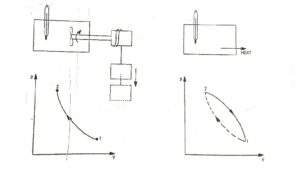
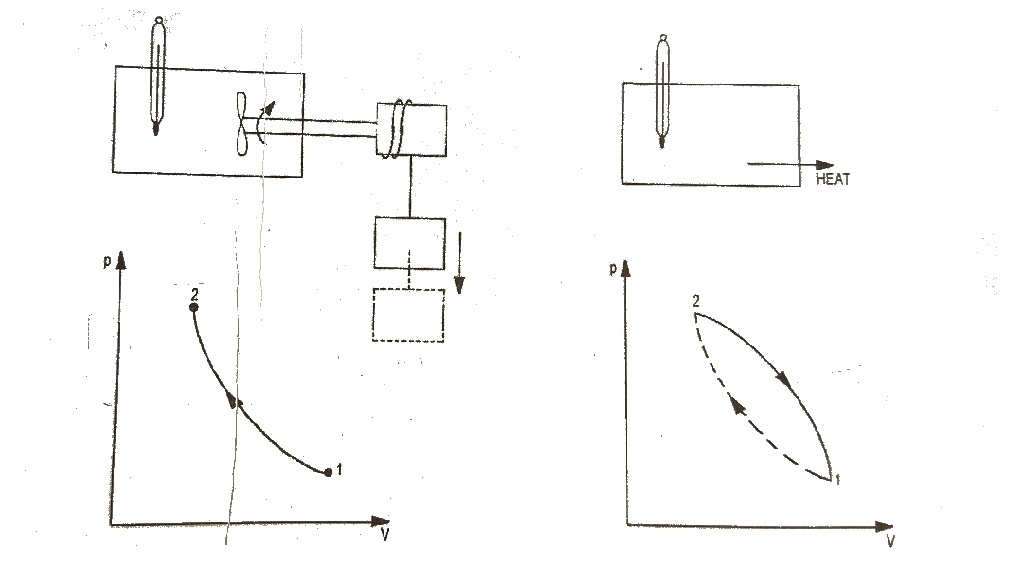
To illustrates this, consider the gas as a system in the container as shown in Fig.1. Let this system go through a cycle that is comprised of two processes. In the first process (1 – 2) the work is done on the system and the temperature of system increased from t₁ to t₂. Let the system then returned to its initial state i.e., to a temperature t₁ by transferring heat from the system until the cycle has been completed. This process is represented by path 2 – 1. When the amount of the work and heat were compared, it was found these two quantities (expressed in consistent units) are equal. This forms the basis of first law of thermodynamics which can be expressed in the form of equation.
∮ dQ = ∮ dW ……..(i)
where ∮ dQ = cyclic integral of heat transfer which represents the net heat transfer during the cycle.
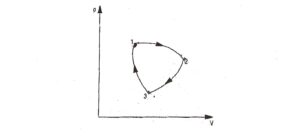
∮ dW = cyclic integral of work transfer which represent net work transfer during the cycle. Consider a cycle as shown in Fig.2. The first of thermodynamics can be expressed as
Q₁₂ + Q₂₃ + Q₃₁ = W₁₂ + W₂₃ + W₃₁ …..(ii)
where the symbol Q and W with their subscripts represent the amount of heat transfer and work transfer for each of the processes making up the cycle. Thus, from equations (I) and (ii).
Mechanical Equivalent of Heat : Joule’s Experiment
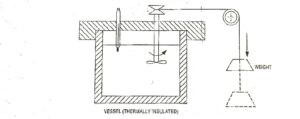
The first law of thermodynamics was formulated as a result of experiments carried out (1840 – 1849) by James Prescott joule An English Physicist.
 |
| Fig.3 Joule’s Experiment |
In one of the experiment, he used water as a system. Work is transferred to the system by means of paddle wheel driven by falling weight as shown in Fig.3. Due to transfer of work, the temperature of water rises.
Now the thermal insulation is removed, and the heat is rejected from the system until it restores the initial state i.e., the temperature water decreases to initial value. Thus, the system has undergone a cycle. Similar experiments by Joule led to an important conclusion that the net amount of heat removed from the system is proportional to the net amount of work transferred into the system.
Thus, ∮ dQ = J∮ dW
where J = Joule’s equivalent (constant) or mechanical engineering of heat
In SI system both ∮ dQ and ∮ dW are expressed I joule. Therefore, the Joule’s constant is unity i.e., J = 1Nm/s
In MKS System ∮ dW is expressed in kgm and ∮ dQ is in kcal, then the value of J is 427, i.e., J = 427 kgm/kcal.

You described the first Law of Thermodynamics in a very easy way. Many students find physics topics related to thermodynamics, magnetism, and electricity very tough. Your explanation not only will help students but also teachers in preparing lectures for their Physics tuition. There is an interesting history of Joule and his struggles. Joule was long ignored by the scientific establishment. He did not have formal schooling but received some tutoring from scientist John Dalton, pioneer of the theory of atomic weights and the composition of molecules. As an adult Joule became the manager of the family business, he worked a full day making beer and then pursued his scientific investigations at the end of the day, as an avocation. Other many scientists were also working and combined with the results of other researchers, Joule’s determination of the mechanical equivalent of heat led to the First Law of Thermodynamics. Your post is going to help many research aspirants. Thanks for sharing.