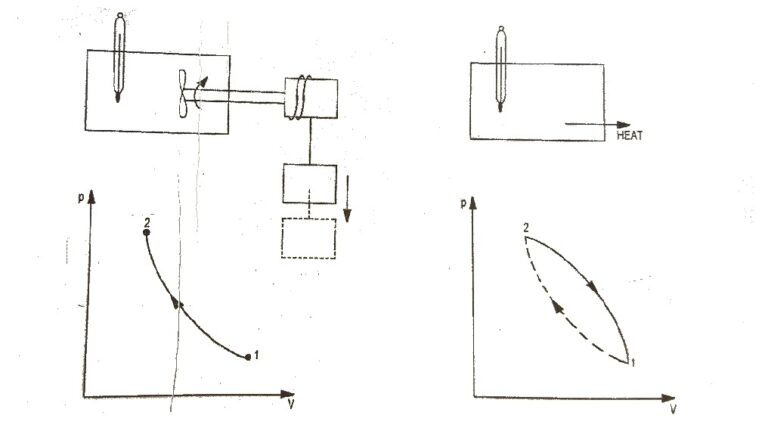
Reversible and Irreversible processes
A process which can be reversible i.e., operated in opposite direction from any state during the process such that the system returns to its initial state and their may be no effect on the surroundings. Thus at the end of the process both the system and surroundings may be restored to their initial state. During reversible process the system passes through a series of equilibrium states. Therefore a reversible process coincides with a quasi-static process. A reversible process is illustrated in Figure the process A-B is a reversible process because in the reverse process B-A the system following the same path and returns to its initial state.
 |
Fig 1. Reversible process Fig 2.Irreversible process
|
A process is reversible when it is carried out very slowly to retain equilibrium condition throughout and should be frictionless
A reversible process is a limiting case which can be approached but not released the process that are not reversible are called Irreversible processes. The process that is carried out with a finite gradient in an Irreversible process. During a Irreversible process the system passes through non-equilibrium states.
Therefore is a Irreversible process is not a Quasi static process and is represented with dotted line joining initial and final states as shown in fig 2
Causes of irreversibility
factors which contribute for irreversibility include friction unresisted expansion (free expansion) heat transfer with a finite temperature difference and combustion.