What is Entropy ∣ Unit of Entropy
What is entropy
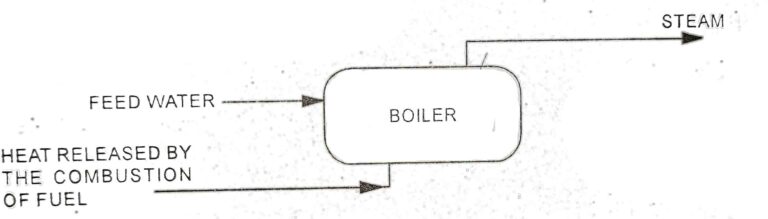
Heat transfer to a system increases the disorder energy (energy due to random distribution of molecules) of the system. Heat transfer from a system reduces the disorder energy. Entropy is a measure of the state of disorder of the system. It increases by addition of heat and decreases by removal of heat from the system.
More about entropy
Entropy is a function of a quantity of heat which shows the possibility of conversion of that heat into work. The increase in entropy is small when heat is added at a high temperature and is greater when heat heat addition is made at lower temperature. Thus for maximum entropy, there is minimum availability for conversion into work and for minimum entropy there is maximum availability for conversion into work
Unit Of Entropy
The SI unit for Entropy (S) is Joules per Kelvin (J/K).