Temperature
Temperature is a measure of hotness or coldness. Based on sensation temperature may be expressed qualitatively as hot warm or cold. However, it will not give exact numerical value to measure temperature. Fortunately, several properties of materials change with temperature in a repeatable and predictable way. This forms basis for accurate measurement of temperature and temperature scales provide a common basis for temperature measurement.
In otherwords
The temperature is a thermal state of a body which distinguishes a hot body from a cold body.
The temperature of a body is proportional to the Stored molecular energy i.e., the average molecular kinetic energy of the molecules in a system. (A particular molecules doesn’t have a temperature it has energy. The gas as a system has temperature).
Instruments for measuring ordinary temperatures are known as thermometer and those for measuring high temperature are known as pyrometers.
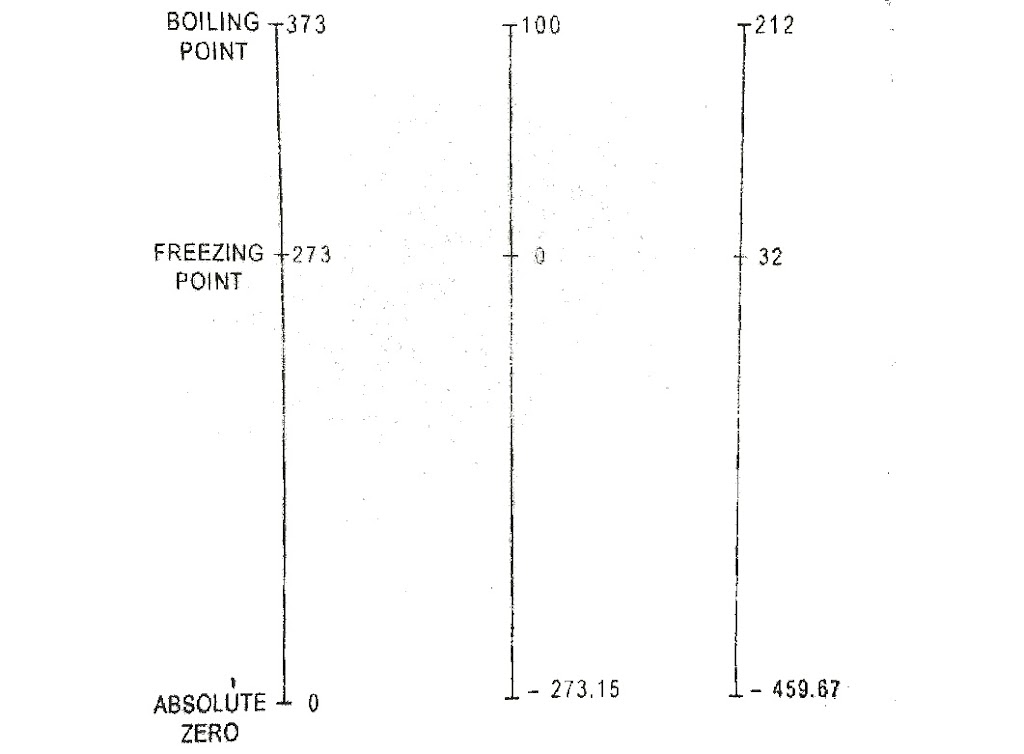
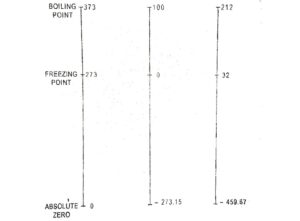
It has been found that a gas will not occupy and volume at certain temperature. This temperature is known as absolute zero temperature. The temperature measured with absolute zero as basis are called absolute temperatures. Absolute temperature is stated in degree centigrade. The point of absolute temperature is found to occur at 273⁰C (app.) below the freezing point of water. Then : Absolute temperature = ⁰C + 273.
Absolute temperature is degree centigrade is
known as degrees Kelvin, denoted by K (SI unit)
Thermometry-thermometric property :
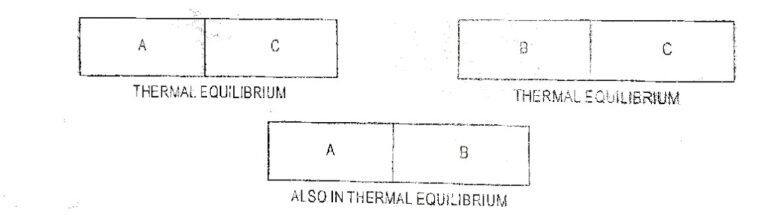
Thermometry is the science of measuring temperature. The principle of thermometry states that when two bodies are in contact and in thermal equilibrium then they are at the same temperature.
In thermometry the change in properties of a substance may be taken as a measure of temperature. The selected property is called thermometric property, and the device used to determine the temperature is called thermometer. An example is the contraction or expansion of mercury column in a glass thermometer as it is cooled or heated. The mercury which has the thermometric property is called thermometric substance.
Different types of thermometers which their own thermometric property are shown in table 1
Best view in desktop mode
|
Thermometer
|
Thermometric
Property (symbol)
|
|
1.
2.
3.
4.
5.
|
Mercury-in-glass thermometer
Constant volume gas thermometer
Constant pressure gas thermometer
Electrical resistance thermometer
Thermocouple
|
Length (L)
Pressure (p)
Volume (V)
Resistance ®
Thermal e.m..f (ε)
|
Table 1
Ice point and steam point
Temperature scales are based on freezing or boiling point of water at atmospheric pressure. These points are also known as ice point and steam point. The ice point is the freezing temperature of pure water at a standard atmospheric pressure. At ice point Ice and water are in equilibrium at atmospheric pressure. The steam point is the temperature at which pure water boils at atmospheric pressure. At steam point. Water and vapour are in equilibrium at atmospheric pressure.
Temperature scales :
Temperature scales are calibrated between Ice and steam point and in Celsius scale (named after the Swedish astronomer A. Celsius), the temperature of ice point is designated as 0⁰C that of a steam as 100⁰C. The length between two points may be divided into 100 equal divisions each division being a Celsius degree.
The ice point and steam point on the Fahrenheit scale (named after German instrument maker G .Fahren heit) are designated has 32°F and 212°F respectively. The length between these point may be divided into 180 equal divisions each division represents a degree Fahrenheit.
Kelvin scale (named after Lord Kelvin) is the absolute scale based on lowest attainable temperature (-273.15). This lowest attainable temperature is called absolute zero.
The temperature scale are related by the following equations :
t°(F) = 1.8 t°(C) + 32
t°(C) = T(K) – 273.15
 |
| Fig.1 |
Limitations of liquid thermometers
The liquid (mercury) thermometer give an arbitrary scale of temperature and need many corrections. It is not suitable for precision work because of its least accuracy is 0.05°C.
Calibration of temperature scale:
In establishing a temperature scale it is necessary to clarify the relation between temperature and thermometric property in order to make calibration. Consider the thermometric property (x) which is a linear function of temperature (t) :
t = a + bx ………(i)
For celsius scale, assume :
ice point, xi = 0
Steam point, xs = 100
Then (xs – xi) is 100 degrees or intervals
Substituting these values in equation (i)
0 = a + bxi ……..(ii)
100 = a + bxs ……..(iii)
Solving equations (ii) and (iii)
a = -{100xi/(xs – xi)}, and b = 100/(xs – xi)
Thus temperature t is given by
t°c = -{100xi/(xs – xi)} + {100/(xs – xi)}x = 100 [(x – xi)/(xs – xi)] …….(iv)
For Fahrenheit scale, xi = 32 and xs = 212 and there exist 180 Intervals between steam and ice points. Substituting evaluated values of ‘a’ and ‘b’ in equation (i)
t°F = [(x – xi)/(xs – xi)] × 180 + 32 …….(v)
Note :
- In thermodynamic calculations, the absolute zero temperature is taken as -273°C.
- It is often necessary to use the absolute scale of temperature other than the conventional temperature in Thermodynamics.
- The magnitude of 1K and 1°C are identical. When a temperature difference is to be evaluated the same result will be obtained when the two temperatures involved are in absolute or celsius.
- In uses, the work degree is omitted with kind. Only the kelvin is used to represent the unit of Temperature.