State ,Path and Process in Thermodynamics | Thermodynamics Cycle
State, path and process
State
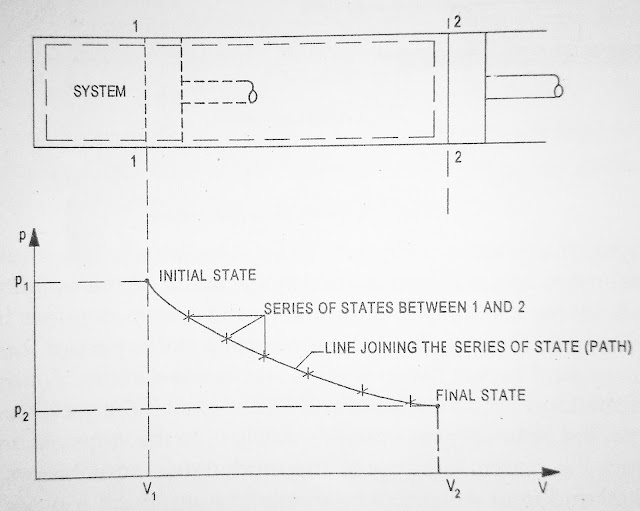
A state is condition of system and is specified by its properties. At a given state all the properties of a system have definite values
Path
Change of state of a system is the consequence of any operation in which properties will change. The series of states through which system passes during a change of state is called the part of the process
Process
A process may be defined as a change of a system from one state to another state. An equilibrium process is a process during which a system remains in equilibrium while its state changes if the system does not remain in equilibrium while it state change then it is called a non-equilibrium process
Thermodynamics cycle
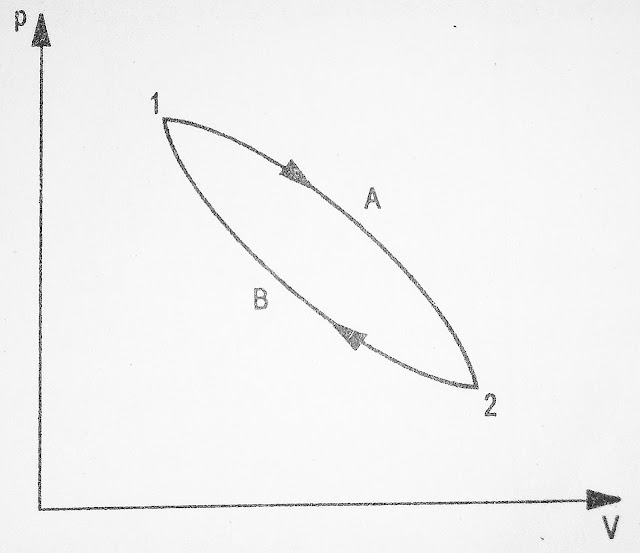
A thermodynamic cycle is defined as a series of process such that the system returns to its initial state. Thus the series of processes (cycle process) in a cycle starts and ends at the same state of a system.
Fig 1.7 illustrate the cycle comprising two processes A and B.

Physics is a really difficult subject and studying such a subject online is not easy. I appreciate the author's efforts in writing these educational articles. A-Level Physics Past Papers can help students to prepare well for exams. Thank you for sharing this. Great blog.
Great article by the great author, it is very massive and informative but still preaches the way to sounds like that it has some beautiful thoughts described so I really appreciate this article.Thermodynamics MCQ