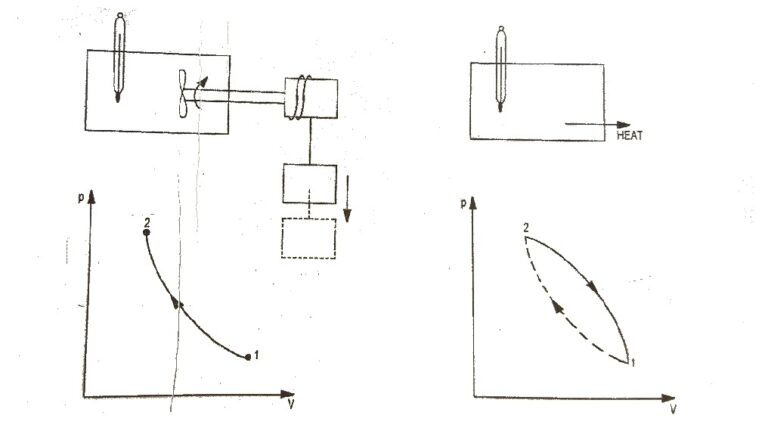
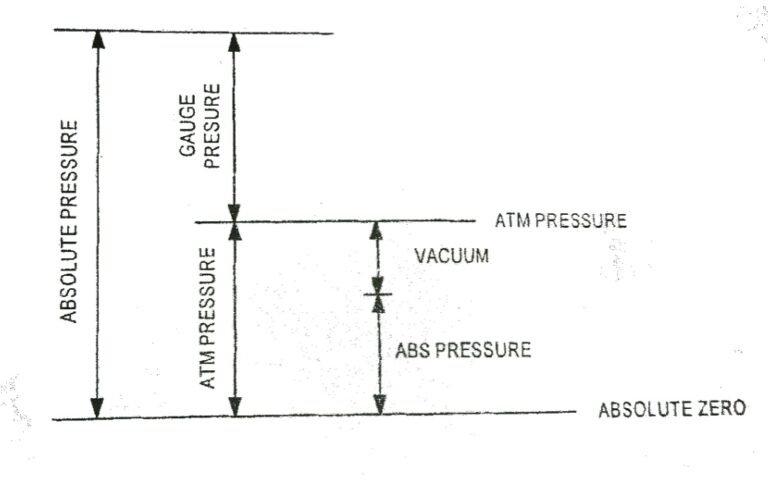
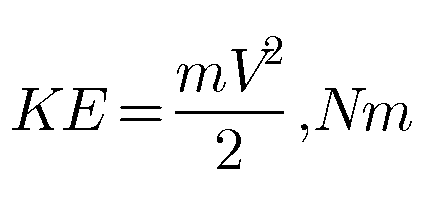First Law for a Closed System Undergoing a Quasi-static process
First Law for a Closed System Undergoing a Quasi-static process In many occasions it is necessary to consider a system undergoing a process rather than a cycle. The equation, ∮ dQ − ∮ dW = 0 is applicable during the system undergoing a cycle, and algebraic sum of all energies transfer across the system boundary…