Pressure
The molecules of a gas are in random motion. The rapidly moving molecules continually impact on the surface of the container and its effect is to produce a force over the surface. The force normal to unit area of surface is called pressure acting on the surface.
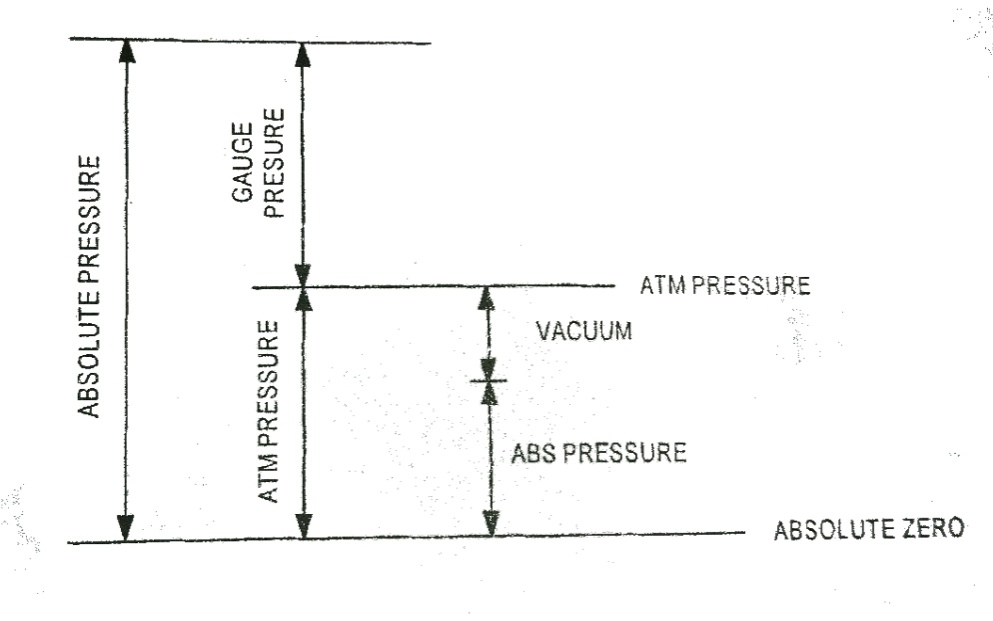
The normal force exerted by the atmosphere on a unit surface area is called atmospheric pressure. Most instruments indicate pressure above the atmosphere. The pressure measured above atmospheric pressure is called gauge pressure. The pressure less than atmospheric pressure is called vacuum. It is the amount by which the pressure inside the container is less than that of surrounding atmosphere.
The the pressure measured above the absolute zero (perfect vacuum) is called absolute pressure.
Absolute pressure = Atmospheric pressure + gauge pressure
In case of vacuum
Absolute pressure = Atmospheric pressure – vacuum
Fig. 1 shows the relation between pressure.
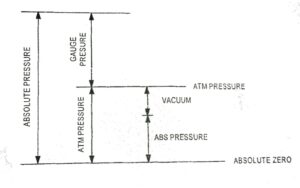 |
| Fig. 1 Pressure Realation |
Unit of Pressure :
The SI unit of pressure is Pascal (Pa) which is equal to a force of 1 N acting on area of 1 m2
1 Pa = 1N/m2
However, Pa is very small unit it is convenient to use multiple of Pascal; kiloPascal, megapascal and gigapascal.
1 kPa = 103(N/m2) ; 1 MPa = 106(N/m2) ; 1 GPa = 109(N/m2) ;
Two additional units of pressure are used extensively. These are bar and atmosphere (atm)
1 bar = 105(N/m²) = 10²(kN/m²)
1 atm = 760 mm Hg = 1.01325 bar
A unit operation commonly used in experimental work at low pressure is 1Torr and is defined as the pressure produced by 1 mm of Mercury column
∴ 1 Torr = 0.001333 bar
Pressure measuring devices:
A very common pressure measuring device is the Bourdon pressure gauge. It consists of a coiled elliptical tube.
One end of the tube is connected to the vessel and other end is closed and connected to the pointer through the gear and level mechanism. The pressure inside the tube tends to deflect the closed end of the tube. This moves a pointer on a calibrated scale to give the value of pressure inside the vassel. It records pressure above the atmosphere, therefore it is a gas pressure
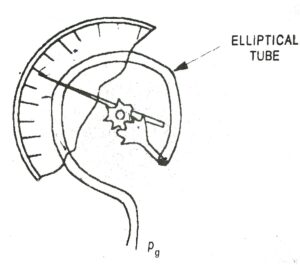 |
| Fig. 2 Bourdon pressure guage |
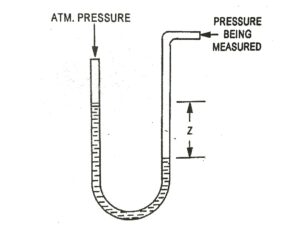 |
| Fig. 3 U-tube manometer |
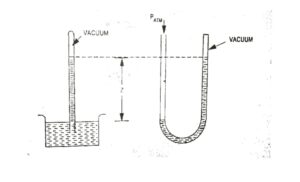 |
| Fig. 4 Barometer |
Note : Most pressure measuring instruments measure the difference between the pressure of a fluid (absolute pressure) and the pressure of the atmosphere (atmosphere pressure). This pressure difference is called gas pressure. Then the absolute pressure of fluid is obtained by the relation.
pabs = patm + pg
Volume
The space occupied by the system is called volume. The volume is expressed in m3 .The other unit of volume is litre.
1 litre = 10-3m3
Specific volume and density:
specific volume is defined as the volume (V) per unit mass (m) of the substance.
Specific volume, v = (V/m) m3/kg
Density is defined as the mass per unit volume.
Density, ρ = (m/V)kg/m3
Thus the specific volume is reciprocal of density
v = 1/ρ = 1/(m/V)