Union of sets
Union of sets
|
Fig(iv)
|
|
Fig(iv)
|
Introduction about thermodynamics Energy is the capacity to do work energy cannot be created or destroyed but only can be changed into other forms (principle of conservation of energy). Thermodynamics mainly deals with interaction between heat and work (mechanical energy) and change in the property is associated with these interactions. The interaction between heat and…
Reversible and Irreversible processes A process which can be reversible i.e., operated in opposite direction from any state during the process such that the system returns to its initial state and their may be no effect on the surroundings. Thus at the end of the process both the system and…
SI (System of international) system of units The SI system consists of seven basic units and two supplementary units. These are represented in table 1 . The unit for other quantities are derived from the basic units which are given in table 2. It is convenient to use prefix to avoid the use of very…
First Law for a Closed System Undergoing a Quasi-static process In many occasions it is necessary to consider a system undergoing a process rather than a cycle. The equation, ∮ dQ − ∮ dW = 0 is applicable during the system undergoing a cycle, and algebraic sum of all energies transfer across the system boundary…
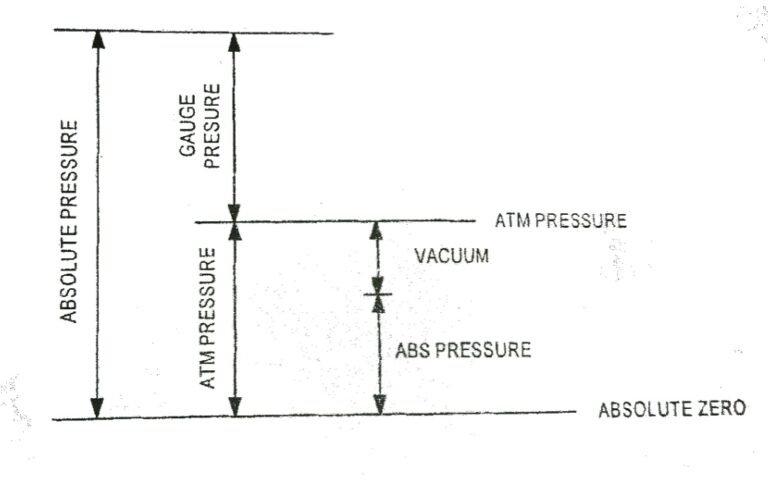
Pressure The molecules of a gas are in random motion. The rapidly moving molecules continually impact on the surface of the container and its effect is to produce a force over the surface. The force normal to unit area of surface is called pressure acting on the surface. The normal force exerted by the atmosphere…
Subsets of the set R of real numbers Following sets are important subsets of the set R of all real numbers: (i) The set of all natural numbers N = { 1, 2, 3, 4, 5, 6,…. } (u) The set of all integers Z = { … – 3, – 2, -1,…