First Law of a Closed System Undergoing a Cycle
First Law of a Closed System Undergoing a Cycle :
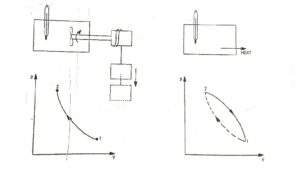 |
| Fig.1 First Law of Thermodynamics |
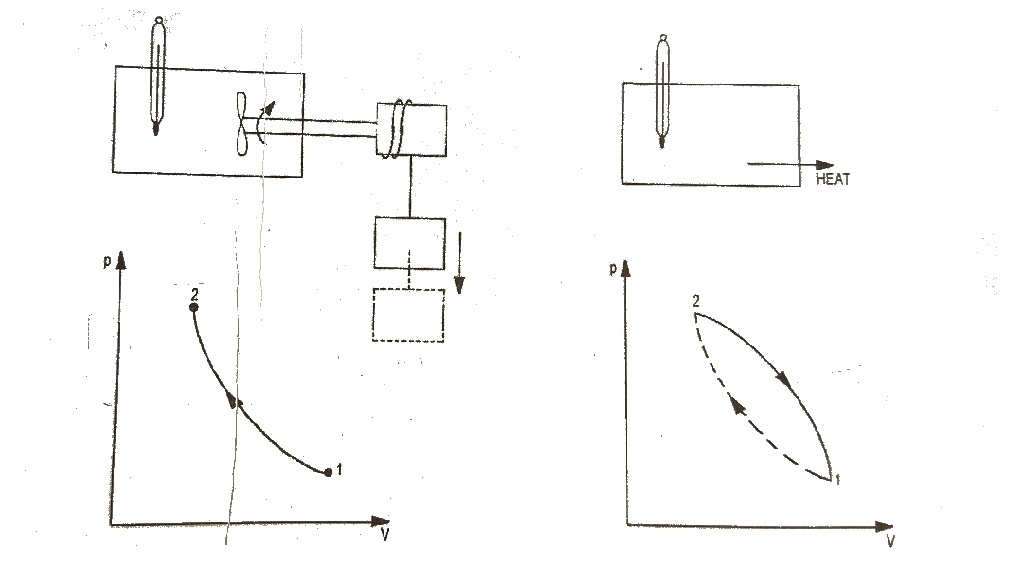
 |
| Fig.1 First Law of Thermodynamics |
Laws of algebra of set THEOREM 1 (Idempotent Laws) For any set A (i) A ∪ A = A (ii) A ∩ A = A PROOF (i) A ∪ A= { x : x ∈ A or x ∈ A} ={x : x ∈ A} = A (ii) A ∩ A = {x : x…
Difference of sets Let A and B be two sets. The difference of A and B written as A – B, is the set of all those elements of A which do not belong to set B Thus A – B={ x : x ∈ A and x ∉ B} or A – B={ x…
Conservation of Energy In the early part of 19th century the scientist developed the concept of energy and hypothesis that it can be neither created nor destroyed ; this come to be known as the law of the conservation of energy. The first law of thermodynamics is merely one statement of this…
Thermodynamic equilibrium Thermodynamic state is a condition of a system and is specified by its thermodynamic properties. Thermodynamic Equilibrium of a system is a state such that if the system is isolated from its surroundings no change in thermodynamic properties would occur. A system will be in a state of thermodynamic equilibrium if the conditions…
State, path and process State A state is condition of system and is specified by its properties. At a given state all the properties of a system have definite values Path Change of state of a system is the consequence of any operation in which properties will change. The series of states through which system…
Energy interaction (Transition) An energy interaction or transition is said to occur or to exist between two systems when one system influences a sustains the state of the other system. Thermodynamics mainly studies the interactions between heat and work and associated property change of the system In otherwords Energy…
helpful content.
Your blog is very valuable which you have shared here about low cost exercise cycle I appreciate your efforts which you have put into this article and also it is a gainful article for us. Thank you for sharing this article here.