Macroscopic and Microscopic Approaches | Concept of continuum
Macroscopic and Microscopic Approaches
The behaviour of matter can be studied by two approaches
1. Macroscopic approach, 2. Microscopic approach
The behaviour of matter can be studied by two approaches
1. Macroscopic approach, 2. Microscopic approach
Intersection of sets Let A and B be two sets. The intersection of A and B is the set of all those elements that belongs to both A and B. See in Fig(v) shaded region show A∩B We denote A intersection B by notation “A ∩ B” Thus A∩B = { x : x ∈…
State, path and process State A state is condition of system and is specified by its properties. At a given state all the properties of a system have definite values Path Change of state of a system is the consequence of any operation in which properties will change. The series of states through which system…
Range of relation Let R be a relation from a set A to a set B. Then the of all second components or coordinates of the ordered pairs belonging to R is called the range of R. Thus, Range of R = { b : (a, b) ∈ R} Clearly, range of R ⊆ B…
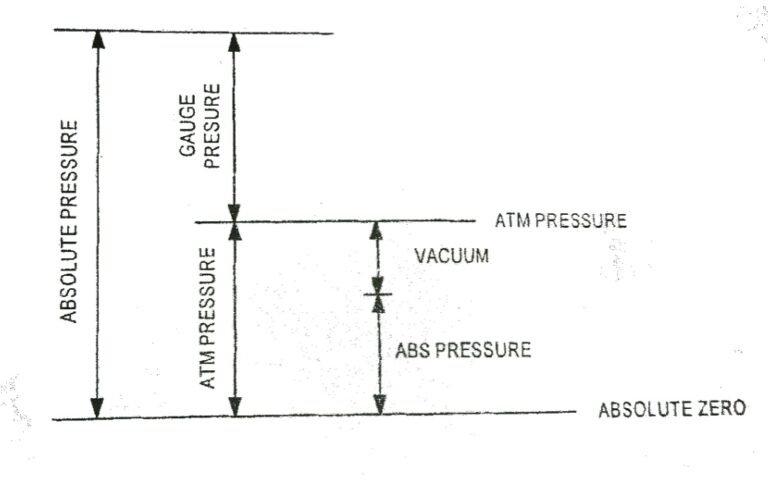
Pressure The molecules of a gas are in random motion. The rapidly moving molecules continually impact on the surface of the container and its effect is to produce a force over the surface. The force normal to unit area of surface is called pressure acting on the surface. The normal force exerted by the atmosphere…
Quasi-static process A process during which the system remains nearly close to an equilibrium state is called Quasistatic process. In other words departure of the state of a system from Thermodynamic Equilibrium state will be Infinitesimally small. Quasi-static process Consider a system as shown in figure the system initially is in equilibrium state as weights…
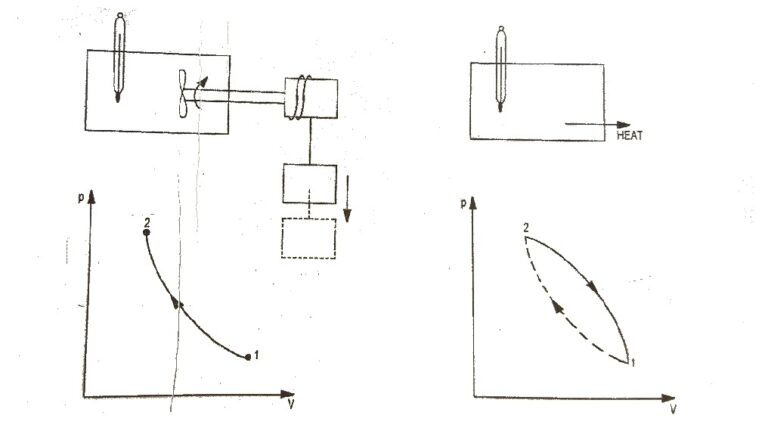
First Law of a Closed System Undergoing a Cycle : The first law of thermodynamics states that during any cycle a system undergoes the cyclic integral of heat is equal to the cyclic integral of the work. Fig.1 First Law of Thermodynamics To illustrates this, consider the gas as a system in…